MISSION
The mission of the Molecular Biology and Viral Oncogenesis Unit is primarily dedicated to the study of virus-associated solid tumors, such as genital/extragenital neoplasms and papillomavirus (HPV), hepatocellular carcinoma, and hepatitis viruses (HBV and HCV). Current projects focus on characterizing the molecular mechanisms of neoplastic transformation, such as genetic and epigenetic alterations of the viral and cellular genome and the associated biological processes. The main research activities aim to identify biomarkers for cancer risk monitoring, early diagnosis, and the development of biomolecules for targeted treatment of virus-associated neoplasms. The activities are structured as follows:
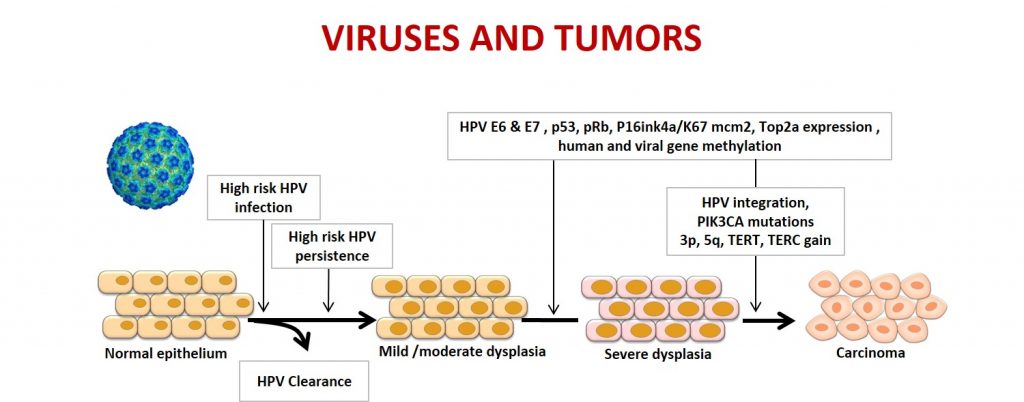
- Study of cellular and viral genetic profiles of lower genital tract and head-neck tumors to identify new markers associated with HPV persistence, tumor progression, and response to therapies. Our studies have contributed to demonstrating that somatic mutations are differently distributed in tumor subtypes and that alternative splicing of viral pre-mRNAs produces specific oncoprotein isoforms in different tumor histotypes.
- Analysis of tumor biomarkers, including circulating ones, in hepatic neoplasms to optimize diagnosis in patients chronically infected with HBV/HCV, for proper staging, prognostic evaluation, and therapeutic responsiveness. The pattern of immune response to specific viral epitopes has led to the discovery of potential tumor risk markers in subjects chronically infected with HCV.
- Development of interfering molecules to counteract the oncogenic action of viruses to inhibit their transforming effect on chronically infected cells. Our group has taken a leading role in developing a structure for the production of bioactive molecules under GMP (Good Manufacturing Practices) standards for the immediate evaluation of the efficacy of new therapies in clinical trials
RESEARCH
The research activity is primarily focused on identifying biomarkers for cancer risk monitoring, early diagnosis, and the development of biomolecules for targeted treatment of virus-associated neoplasms. The active projects at the Molecular Biology and Viral Oncogenesis Unit are as follows:
2020– 2024 An integrated approach to define genetic and molecular mechanisms involved in the pathogenesis of lower female genital tract tumours for early diagnosis as well as for discovery of progression biomarkers and new therapeutic targets (RF-2018-12366163). The project is focused on three main objectives: 1) identifying new cellular and viral diagnostic markers for cervical and vulvar tumors; 2) developing alternative protocols to animal use through the preparation of complex organoids; 3) identifying new pharmacological targets based on cellular and viral biomarkers.
2022 – 2024 Virus-Related Oncogenic Tumors: New Biomarkers for Risk Assessment and Early Diagnosis (VIRTUMO, L1/10 ID 2772898). The aim of the study is to define new diagnostic and prognostic markers in tumors attributable to infectious agents by studying molecular signatures related to viruses and risk factors such as smoking and alcohol in tissues and fluid matrices
2022– 2027 Unravelling and targeting non-canonical functions of telomerase in squamous cell carcinoma of female lower genital tract (AIRC_IG_2021_ID_26111). The project aims to characterize the effect of TERT promoter (TERTp) mutations on telomerase expression and the activation of non-canonical pathways in squamous cell carcinomas of the female genital tract.
2023– 2025 Identification of common pathogenic mechanisms driving squamous cell carcinomas of the anogenital tract and head&neck region to develop overarching therapeutic strategies (PNRR-MAD-2022-12376570). The study is aimed at characterizing the oncogenic pathways of telomerase and sirtuin 1 in squamous cell carcinomas of the ano-genital tract and head-neck region to evaluate new targeted therapeutic combinations
2024-2026 Characterization of immune genotypes and antibody profiles to foster the discoVERY of diagnostic bioMARKERS of liver cancer development – VERYMARKERS – (PNRR-MCNT2-2023-12377164). The INT working group analyzes the immuno-serological profile of HCV-positive patients with chronic infection or HCV-related neoplasms against a panel of peptides to evaluate the relationship between antibody levels and the risk of developing liver tumors.
2024-2026 Unraveling the Molecular and Immunologic Mechanisms of Intrahost Persistence in Emerging and Re-Emerging Arboviral Infections – GENESIS – (INFACT COC-1-2023-UNIPV S1.P0002). The INT working group, within the framework of WP4, is involved in immuno-serological profiling to identify biomarkers of disease progression associated with TOSV infection.
2024-2026 Analisi di biomarcatori in biopsia liquida per la diagnosi precoce di neoplasia orofaroingea, ginecologica e anale associata ad HPV – BIOVIRUS – (Progetto 5x mille 5M-2022-23685451). The study is based on the molecular characterization of the HPV virus in various biological matrices and the antibody response to early proteins E6 and E7 to identify innovative circulating biomarkers in HPV-related tumors..
PERSONNEL
Acting Director
Maria Lina Tornesello (Biologist) 08117770588 m.tornesello@istitutotumori.na.it (ORCID: 0000-0002-3523-3264)
Staff Scientists
Andrea Cerasuolo (Biologist) 08117770590 a.cerasuolo@istitutotumori.na.it (ORCID: 0000-0002-6410-7515)
Patrizia Bonelli (Biologist) 08117770599 p.bonelli@istitutotumori.na.it (ORCID: 0000-0002-0916-4015)
Franca Maria Tuccillo (Biologist) 08117770598 f.tuccillo@istitutotumori.na.it (ORCID: 0000-0003-4167-2535)
Researchers
Noemy Starita (Biotechnologist) 08117770589 n.starita@istitutotumori.na.it (ORCID: 0000-0002-5169-7640)
Researchers (PNRR)
Sara Amiranda (Biotechnologist) 08117770601 sara.amiranda@istitutotumori.na.it (ORCID: 0000-0003-3995-3864)
Luisa Dassi (Biologist) 08117770601 luisa.dassi@istitutotumori.na.it (ORCID: 0000-0002-2350-1997)
Tiziana Pecchillo Cimmino (Biotechnologist) 08117770601 tiziana.pecchillocimmino@istitutotumori.na.it (ORCID: 0000-0003-0545-2981)
Laboratory Technicians
Mariapia Napolitano 08117770601 mpia.napolitano@istitutotumori.na.it
Fellows
Maria Bruno (Biologist) 08117770601 maria.bruno@istitutotumori.na.it
Gaetano Della Volpe (Biotechnologist) 08117770601 gaetano.dellavolpe@istitutotumori.na.it
Salvatore Gagliarde (Biologist) 08117770601 salvatore.gagliarde@istitutotumori.na.it
MAIN SCIENTIFIC PUBLICATIONS
Tornesello ML. TP53 mutations in cancer: Molecular features and therapeutic opportunities (Review). Int J Mol Med. 2025 Jan;55(1):7. doi: 10.3892/ijmm.2024.5448. Epub 2024 Oct 25. PMID: 39450536; PMCID: PMC11554381.
Santoro A, Angelico G, Arciuolo D, Scaglione G, Padial Urtueta B, Aquino G, Starita N, Tornesello ML, Rega RA, Pedicillo MC, Mazzucchelli M, Stefano IS, Zamparese R, Campisi G, Mori G, Zannoni GF, Pannone G. TLR4 Downregulation Identifies High-Risk HPV Infection and Integration in H-SIL and Squamous Cell Carcinomas of the Uterine Cervix. Curr Issues Mol Biol. 2024 Oct 10;46(10):11282-11295. doi: 10.3390/cimb46100670. PMID: 39451550; PMCID: PMC11506170.
Tornesello AL, Cerasuolo A, Starita N, Amiranda S, Cimmino TP, Bonelli P, Tuccillo FM, Buonaguro FM, Buonaguro L, Tornesello ML. Emerging role of endogenous peptides encoded by non-coding RNAs in cancer biology. Noncoding RNA Res. 2024 Oct 28;10:231-241. doi: 10.1016/j.ncrna.2024.10.006. PMID: 39554691; PMCID: PMC11567935.
Giorgi-Rossi P, Tornesello ML, Buonaguro FM. Why so much uncertainty about adjuvant HPV vaccines after local treatment? Can the discrepancy between the positive statistical results and the scientific community doubts be solved? Infect Agent Cancer. 2024 Apr 4;19(1):11. doi: 10.1186/s13027-024-00572-9. PMID: 38575999; PMCID: PMC10996102.
Cavalluzzo B, Viuff MC, Tvingsholm SA, Ragone C, Manolio C, Mauriello A, Buonaguro FM, Tornesello ML, Izzo F, Morabito A, Hadrup SR, Tagliamonte M, Buonaguro L. Cross-reactive CD8+ T cell responses to tumor-associated antigens (TAAs) and homologous microbiota-derived antigens (MoAs). J Exp Clin Cancer Res. 2024 Mar 20;43(1):87. doi: 10.1186/s13046-024-03004-z. PMID: 38509571; PMCID: PMC10953141.
Pubmed list of scientific publications
CLINICAL STUDIES
PNRR‐MAD‐2022 Identificazione dei meccanismi patogenetici alla base dei carcinomi a cellule squamose del tratto anogenitale e della regione testa‐collo per sviluppare strategie terapeutiche condivise. Protocollo CEI n. 9/23 oss – Clinical Trial n. NCT06236464. (Deliberazione n.323 del 05/03/2023).
PROGETTO AIRC – Identificazione ed inibizione delle attività non convenzionali della telomerasi nei carcinomi a cellule squamose del basso tratto genitale femminile. Protocollo CEI n. 33/22 oss. (Deliberazione n.1177 del 02/12/2022).
VIRTUMO – Tumori correlati a virus oncogeni: Nuovi biomarcatori per la valutazione del rischio e per la diagnosi precoce. Protocollo CEI n. 30/22 oss. (Deliberazione n.1177 del 02/12/2022).
Ricerca Finalizzata RF-2018 – Un approccio integrato per definire i meccanismi genetici e molecolari coinvolti nella patogenesi dei tumori del basso tratto genitale femminile per la scoperta di biomarcatori per la diagnosi precoce e di nuovi bersagli terapeutici. Protocollo CEI n. 61/21 oss. (Deliberazione n.268 del 10/03/2022).
HEPATOCHIP – Valutazione della predittività oncologica dell’epatochip per la progressione oncologica di lesioni croniche epatiche associate o meno alle infezioni da HCV. Protocollo CEI n. 51/21 oss. (Deliberazione n.1569 del 11/12/2021).
PARTICIPATION IN OTHER CLINICAL STUDIES
CABOTEM – Studio interventistico di Fase II a singolo braccio per valutare l’attività e la safety della combinazione di Cabozantinib eTemozolomide nelle neoplasie neuroendocrine polmonari (Lung-NENs) e gastro-entero-pancreatiche (GEP-NENs) in progressione dopo SSAs, Everolimus, Sunitinib, chemioterapia e/o PRRT (partecipante all’equipe). (Deliberazione n. 1335 del 17/12/2020).